Equilibrium, Conservation and Kinetic Equations Cards (-RG)
There are three Equations Cards that can be used with the ECKEChem Reactive Transport module: the Equilibrium Equations Card, the Conservation Equations Card, and the Kinetic Equations Card. The details of translating a biochemical/geochemical reaction network into these cards can be found in the manuscript by Fang et al. (2003). The objective here is to generally describe the form and how these cards are used in STOMP/eSTOMP.
Conservation Equations
The Conservation Equations Card is required to execute a reactive transport simulation. Conservation equations define the component species, which essentially are a set of species, whose collective stochiometrically weighted summed concentration is invariant with time:
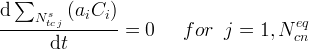
Symbols | |
|---|---|
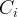 |
concentration of species i, mol/m3 aqu. |
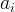 |
conservation equation stoichiometric coefficient of species i |
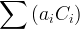 |
component species concentration, mol/m3 aqu. |
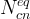 |
number of conservation equations |
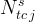 |
number of species in total-component species j |
Equilibrium Equations
Equilibrium equations, often referred to as mass action equilibrium equations, are not required for executing a simulation with ECKEChem. However, if an equilibrium equation is defined, then a corresponding Equilibrium Reactions Card is also required. Since unique equation names are required, the equation names must match between the Equilibrium Equations Card and Equilibrium Reactions Card. If the toEcke pre-processor is used, then the equilibrium equations will be named numerically as EqRc-1...EqRc-n. Equilibrium equations relate species activities through an equilibrium constant:
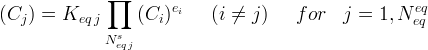
Symbols | |
|---|---|
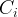 |
concentration of species i, mol/m3 aqu. |
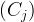 |
aqueous activity of species j, mol/m3 aqu. |
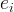 |
stoichiometric exponent of species i |
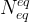 |
number of equilibrium equations |
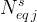 |
number of equilibrium species in equilibrium equation j |
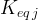 |
equilibrium constant of equilibrium equation j |
The exponents are stochiometric coefficients in the reaction network and the equilibrium constant can be temperature dependent. The equilibrium species (indicated with the subscript i ) is distinguished from the other species in the equilibrium equation (indicated with the subscript j ) by being the first species listed for the equilibrium equation. The primary information required in the Equilibrium Equations Card is the stoichiometry associated with the equation. The general format is to specify the number of species first, followed by the species name and its stoichiometric coefficient, and then to terminate with the equilibrium equation name.
Kinetic Equations
Kinetic equations define kinetic components but are not required to execute an ECKEChem simulation. If a kinetic equation is defined, then a corresponding Kinetic Reactions Card is also required. Since unique equation names are required, the equation names must match between the Kinetic Equations Card and Kinetic Reactions Card. If the toEcke pre-processor is used, then the equilibrium equations will be named numerically as KnRc-1...KnRc-n. Kinetic equations are similar in form to conservation equations, except that the stochiometrically weighted sum of species concentrations vary in time according to a weighted sum of kinetic rates:
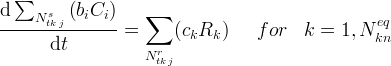
Symbols | |
|---|---|
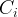 |
concentration of species i, mol/m3 aqu. |
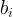 |
kinetic-equation stoichiometric coefficient of species i |
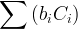 |
kinetic species concentration, mol/m3 aqu. |
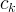 |
kinetic-equation reaction-rate coefficient of reaction k |
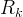 |
kinetic rate of reaction k, mol/s m3 aqu. |
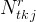 |
number of kinetic reactions associated with total-kinetic species j |
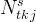 |
number of species in total-kinetic species j |
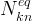 |
number of kinetic equations |
The primary information required in the Kinetice Equations Card is the stoichiometry associated with the equation. The general format is the kinetic component species name, followed by the number of species in the kinetic equation. Stoichiometry is also specified , but additional information is needed on the number of kinetic reactions in the kinetic equation.
References
Fang, Y., G.T. Yeh and W.D. Burgos. 2003. "A general pardigm to model reaction-based biogeochemical processes in batch systems". Water Resources Research 39(4): 1083 - 1107.
ECKEChem Input Cards
- Conservation Equations Card
- Equilibrium Equations Card
- Equilibrium Reactions Card
- Gas Species Card
- Kinetic Equations Card
- Kinetic Reactions Card
- Lithology Card
- Solid Species Card
- Species Link Card
- Aqueous Species Card
- Exchanged Species Card
- Plot File Species Variables
- Reference Node Species Variables
