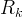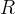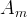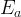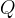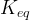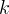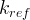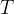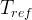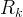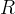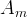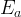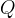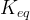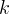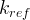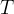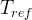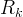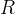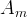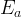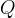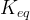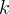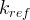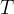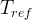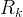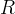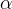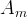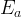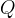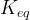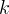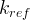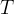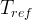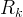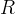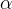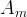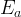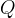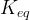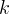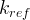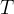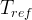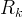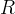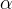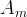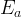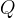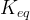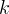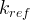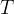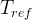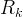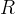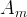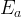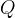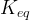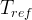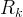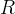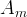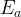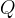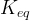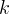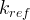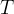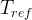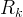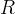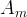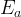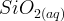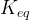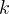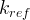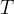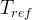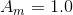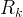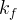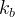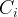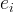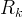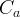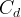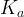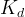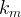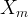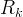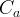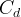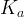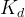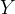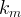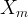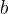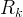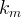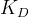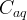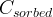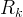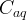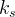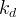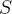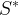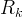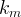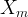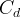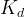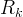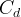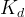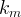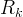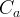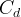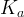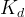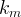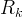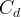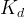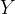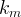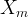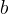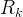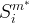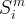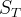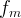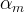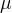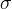Kinetic Reactions Card Options (-R)
Card Options
The following rate formulations can be selected:
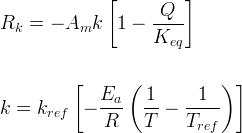
Mineral precipitates when Rk > 0.
Info
|
|||||||||||||||||||||||||||||
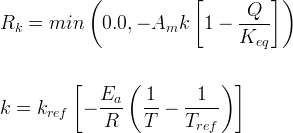
Mineral dissolves when Rk < 0.
Info
|
|||||||||||||||||||||||||||||
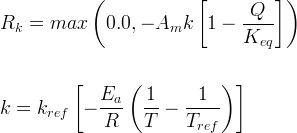
Mineral precipitates when Rk > 0.
Info
|
|||||||||||||||||||||||||||||
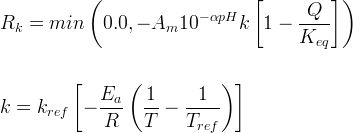
Mineral dissolves when Rk < 0.
Info
|
|||||||||||||||||||||||||||||
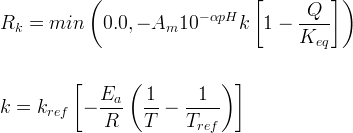
Mineral dissolves when Rk < 0.
Info
|
|||||||||||||||||||||||||||||
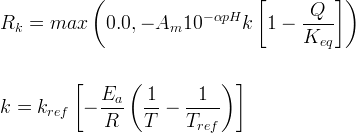
Mineral precipitates when Rk > 0.
Info
|
|||||||||||||||||||||||||||||
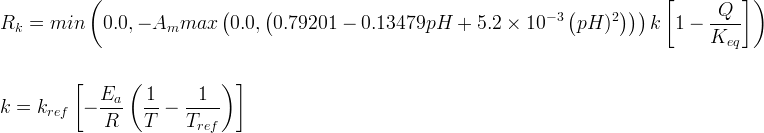
Mineral dissolves when Rk < 0.
Info
|
|||||||||||||||||||||||||||||
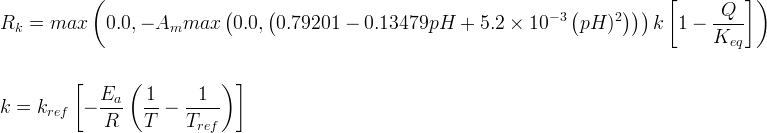
Mineral precipitates when Rk > 0.
Info
|
|||||||||||||||||||||||||||||
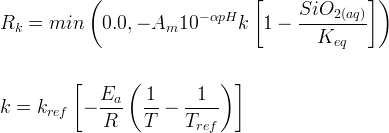
Mineral dissolves when Rk < 0.
Info
|
|||||||||||||||||||||||||||||
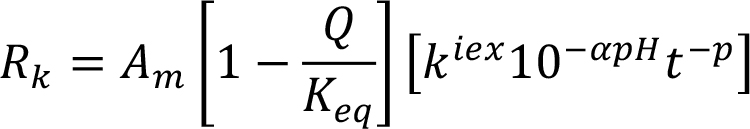
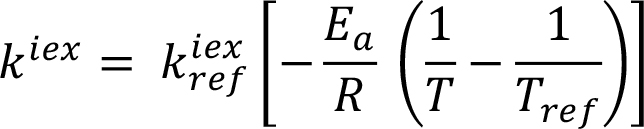
|
|||||||||||||||||||||||||||||
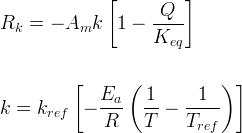
Mineral precipitates when Rk > 0.
Info
|
|||||||||||||||||||||||||||||
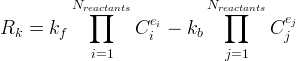
|
|||||||||||||||||||||||||||||
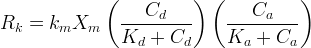
|
|||||||||||||||||||||||||||||
The rate of biomass is represented as: 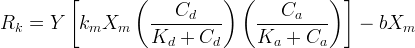
|
|||||||||||||||||||||||||||||
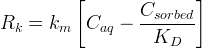
Rate is positive when reaction proceeds to sorption.
|
|||||||||||||||||||||||||||||
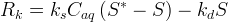
|
|||||||||||||||||||||||||||||
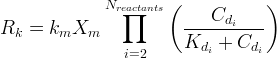
|
|||||||||||||||||||||||||||||
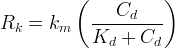
|
|||||||||||||||||||||||||||||
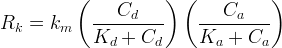
|
|||||||||||||||||||||||||||||
The rate of biomass is represented as: 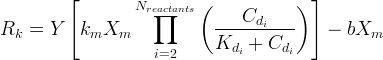
|
|||||||||||||||||||||||||||||
This model is specific for the following uranium surface complexation reactions: 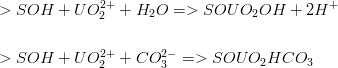
The detailed description of this model is presented in Liu et al.( 2008). 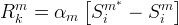
where m indicates the m-th sorption site. The rate constants αm are assumed to follow a lognormal probability distribution. The probability of a site that has a correspondent rate constant of α is defined as: 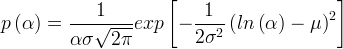
The reaction rate constant for a site m, αm, is determined from the following equation: 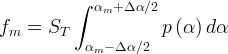
|
|||||||||||||||||||||||||||||
This model is identical in form to the lognormal model, but considers sorption-desorption independently from mobile and immobile domains. |
|||||||||||||||||||||||||||||
References
Liu et al.( 2008). "Scale-dependent desorption of uranium from contaminated subsurface sediments", WRR, 44, W08413.
ECKEChem Input Cards
- Conservation Equations Card
- Equilibrium Equations Card
- Equilibrium Reactions Card
- Gas Species Card
- Kinetic Equations Card
- Kinetic Reactions Card
- Lithology Card
- Solid Species Card
- Species Link Card
- Aqueous Species Card
- Exchanged Species Card
- Plot File Species Variables
- Reference Node Species Variables