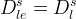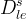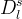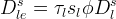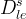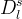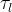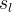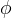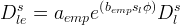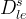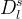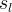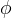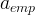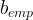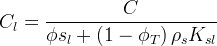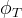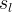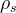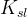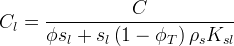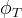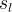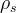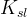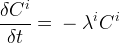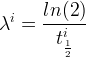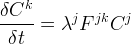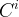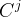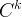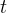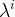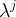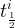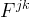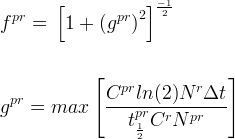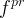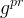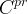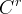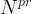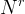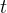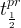Solute/Fluid Interaction Card Options (GT)
Effective Diffusion Options
Gas/Aqueous Partition Function:
Solid/Aqueous Partition Options:
Reaction Options
References
Campbell, GS. 1985. Soil Physics with Basic: Transport Models for Soil-Plant Systems. Elsevier Science Publishers, New York, New York.
Kemper, WD and JC van Schaik. 1966. "Diffusion of Salts in Clay-Water Systems," Soil Sci. Soc. Am. J., 30:534-554.
STOMP User Guide Home
- Simulation Title Card
- Solution Control Card
- Grid Card
- Inactive Nodes Card
- Rock/Soil Zonation Card
- Mechanical Properties Card
- Hydraulic Properties Card
- Saturation Function Card
- Aqueous Relative Permeability Card
- Directional Aqueous Relative Permeability Cards
- Gas Relative Permeability Card
- Thermal Properties Card
- Salt Transport Card
- Solute/Fluid Interaction Card
- Solute/Porous Media Interaction Card
- Initial Conditions Card
- Boundary Conditions Card
- Source Card
- Output Control Card
- Surface Flux Card